Einleitung
Dieses Material ist Teil des Leitfadens Pathologiebefund.
|
Inhaltsverzeichnis
- 1 Einleitung / Introduction
- 2 Foreword
- 3 Introduction
- 4 VOLUME 1 - INTEGRATION PROFILES
- 5 4 Anatomic Pathology Structured Reports (APSR Profile
Einleitung / Introduction
Dieses Dokument ist ein Entwurf für die Umsetzung von Pathologie-Berichten mit Hilfe von HL7 CDA R2. Diese Entwicklung soll die Pathologieintegration innerhalb Deutschlands, im deutschsprachigen Raum und auch international fördern. Sie wird vom Bundesverband Deutscher Pathologen e.V. unterstützt.
Ausgehend vom HL7 V3 Arztbrief-Leitfaden der bvitg (ehemals VHitG) stützt sich der Pathologiebefund auf die IHE-Dokumente "IHE Anatomic Pathology, Technical Framework Supplement, Anatomic Pathology Structured Reports - Trial Implementation, Rev.2.0" vom 06.08.2013, "APSR Value Sets Appendix, Rev. 2.0" vom 06.08.2013, "IHE Anatomic Pathology (PAT), Technical Framework, Volume 1, Revision 2.0 1, Trial Implementation" vom 23.7.2010, "IHE Anatomic Pathology (PAT), Technical Framework, Volume 2 15, Revision 2.0, Trial Implementation" vom 23.7.2010.
Die spezifisch deutschen Belange werden als National Extensions für IHE entwickelt und mit den Aktivitäten von ELGA, Österreich, abgestimmt. Darüber hinaus wird eine enge Kooperation mit IHE-AP und HL7-AP gesucht, um die in Deutschland erarbeiteten Details international einzubringen.
Orientiert wird dabei auf eine möglichst vollständige Berücksichtigung des "Leitfadens Pathologie/Neuropathologie (ehem. TM-30)" des Sektorkomitees Pathologie für die Anwendung der DIN EN ISO/IEC 17020 in der Pathologie/Neuropathologie.
Weiterhin wird angestrebt, die durch den Bundesverband Deutscher Pathologen und die Deutsche Gesellschaft für Pathologie veröffentlichten "Empfehlungen zur pathologisch-anatomischen Diagnostik von Kolorektalen Karzinomen, Mammakarzinomen und Prostatakarzinomen" in HL7 CDA R2 kompatible Templates zur Integration als Checklisten in Pathologie-Management-Systeme umzusetzen.
Auf dieser Basis soll der Import von HL7 CDA R2 Dokumenten von der Pathologie in KIS-Systeme sowie in Tumormeldungen und Qualitätssicherungs- und Tumordokumentationssysteme (z.B. AQUA, MaSC, ix.mid etc.) umgesetzt werden.
This document is a proposal for Anatomical Pathology Structured Reports (APSR)in HL7 CDA R2. It should help to integrate Anatomic and Clinical Pathology in German speaking countries into clinical and research environments by enabling interoperability between a variety of LIS, HIS, and cancer registries as well as elctronic health records. The specific German aspects are constraints of the IHE Anatomic Pathology profiles. They will be developed in close collaboration with the Austrian ELGA initiative. On the other hand, there is a close cooperation with IHE-AP and HL7-AP as to bring in the national demands into international standard development.
For the German APSR the "Guideline Pathology / Neuropathology" (formerly TM-30) of the Sector Committee Pathology for the implementation of DIN EN ISO/EC 17020 shall be regarded. Furthermore, the recommendations of the German Society of Pathology for pathologic-anatomical reporting of colorectal, breast, and prostate cancers should be enabled by CDA compatible codes and value sets.
Foreword
This is a supplement to the forthcoming IHE Pathology and Laboratory Technical Framework. Each supplement undergoes a process of public comment and trial implementation before being incorporated into the volumes of the Technical Frameworks.
This supplement is published as Final Text on xxx, 2016 and may be available for testing at subsequent IHE Connectathons. The supplement may be amended based on the results of testing. It will be incorporated into the Pathology and Laboratory Technical Framework. Comments are invited and may be submitted at http://ihe.net/PaLM_Public_Comments.
This supplement describes changes to the existing technical framework documents and where indicated amends text by addition (bold underline) or removal (bold strikethrough), as well as addition of large new sections introduced by editor’s instructions to "add new text" or similar, which for readability are not bolded or underlined.
"Boxed" instructions like the sample below indicate to the Volume Editor how to integrate the relevant section(s) into the relevant Technical Framework volume:
Replace Section X.X by the following:
General information about IHE can be found at: http://www.ihe.net.
Information about the IHE Pathology and Laboratory Medicine domain can be found at: http://ihe.net/IHE_Domains.
Information about the structure of IHE Technical Frameworks and Supplements can be found at: http://ihe.net/IHE_Process and http://ihe.net/Profiles.
The current version of the IHE Technical Framework (if applicable) can be found at: http://ihe.net/Technical_Frameworks.
Introduction
This supplement is written for Trial Implementation.
This supplement prepares a new volume, Volume 3, of the Pathology and Laboratory Medicine (PaLM) Technical Framework.
This supplement references other documents the reader should be aware of:
1. IHE IT Infrastructure Technical Framework Volume 1
2. IHE IT Infrastructure Technical Framework Volume 3
3. IHE PCC Technical Framework Volume 2
4. HL7 CDA r2 normative edition 2005
5. Goldsmith, J.D., et al., Reporting guidelines for clinical laboratory reports in surgical pathology. Arch Pathol Lab Med, 2008. 132(10): p. 1608-16
6. CAP Cancer Protocols and Checklists 2010
Profile Abstract
Anatomic pathology reports (APR) document the pathologic findings in specimens removed from patients for diagnostic or therapeutic reasons. This information can be used for patient care, clinical research and epidemiology. This Content Profile is the result of a joint initiative from IHE and HL7 anatomic pathology workgroups who brought along a methodology and tools to facilitate the development of consensus-based anatomic pathology structured reports (APSR) and to publish an HL7 Clinical Document Architecture (CDA) implementation guide for these APSR.
Open Issues and Questions
APSR-13 – Missing LOINC code for intraoperative section: This code does not seem to be available in LOINC. The creation will be submitted to the Regenstrief Institute.
Closed Issues
APSR-01 – List of potential sections of an AP structured report:
- Clinical information (content supposedly provided by the ordering physician)
- Intraoperative observations (in case of intraoperative consultation, which may be macroscopic only or may include cytology and/or frozen section)
- Macroscopic observations
- Microscopic observations
- Diagnosis
- Procedure steps (this technical section is also useful for tracking the sequence of operations performed on the specimen at the work area), which does not preclude some of this information from appearing in one of the other sections (e.g., the Macroscopic observations section).
APSR-02 – Content of sections:
- Each section SHALL contain a text element presenting the content to the human reader. The profile does not constrain the layout of this narrative block.
- The Diagnostic Conclusion section SHALL contain an entry element with the corresponding machine-readable data.
- The other sections SHOULD contain an entry element with the corresponding machine-readable data.
- The Clinical information section MAY contain other sections.
APSR-03 – Handling the mix of coded content and free unstructured text: AP reports are often composed of free text (which can be dictated), assembled with a set of coded information (e.g., some AP observations). The Content Creator application must handle these two kinds of content, and provide a user interface, which avoids any confusion between these two kinds of content, both at creation time and update time (e.g., using forms with dedicated free text areas and distinct areas for coded fields).
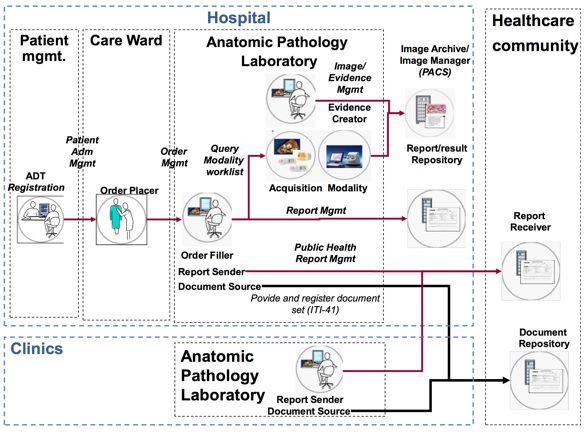
The body of the report is a hierarchy of sections. Each section presents its content in its text element, as human-readable text, possibly illustrated by some embedded images. This human-readable content of the section, or a part of it, may also be present as machine-readable data coded with the appropriate terminologies (e.g., ICD-O-3, PATHLEX, SNOMED CT, LOINC, ADICAP, or any other terminology admitted by this profile or a national extension of it …) in entry elements at the bottom of the section.
There are zero or more entry elements in a section. Each entry element carries the machine-readable data related to a specimen or to a group of specimens (see APSR-10). The entry is then subdivided per problem investigated on the specimen(s) (see closed issue APSR-06 below).
The text element of the section is supposed to reflect the same organization: per specimen or group of specimens, and then, per problem investigated. However, this APSR Content Profile does not explicitly describe the structure of this text element, and leaves it up to the Content Creator applications, or to further constraints brought by national extensions of this profile. The text element of a section in an APSR instance may be a mix of paragraphs, tables, diagrams and images, composed by the author of the report with the sole purpose of clarity and comprehensiveness for the reader.
APSR-04 – Linking AP observations to images/evidence documents: It is sometimes useful to present to the reader of the report the images related to the observations. The CDA AP report provides the CDA R2 standard means to embed images at the observation level or at the organizer level in an entry, using the observationMedia element and potentially the regionOfInterest element. These images can only be small illustrations. Big images – like whole slide images or evidence documents – will stay in their own storage infrastructure, accessible via the DICOM standard protocol, and may be associated with the APSR document, via a DICOM KOS list of references (as described in the XDS-I profile from the Radiology domain), issued in the same submission set.
APSR-05 – Coding of the TNM: The value sets for the TNM of various tumors will be created into the PathLex terminology built by IHE PAT, based on the reference material of this profile. PathLex is a temporary code system, which may be used in an AP structured report, but is not mandated by this APSR content Profile. Alternatively for TNM coding as well as for ICD-O-Typing and Grading specific templates built by IHE PaLM should be used.
APSR-06 – Two (or more) distinct problems observed on the same specimen: In this case, the AP report template should provide a means to group the observations per problem. The solution consists in inserting a battery organizer grouping all observations related to the same problem below the specimen information organizer. See also APSR-03 above.
APSR-07 – Representing the hierarchy of specimens in an entry : The operations on specimen and production of child specimens are tracked in the “Procedure Steps” section, which has a level 3 entry element for that purpose.
APSR-08 – Human authors and/or authoring devices: Do we have use cases for recording authoring devices as “author” in the report or a part of it? Or do we allow only human authors? The answer is “Both”: Image modalities may be authoring devices in some situations.
APSR-09 – Transcriptionist: A transcriptionist may appear in the CDA report in the header as a dataEnterer element, or within an entry (organizer or observation) as a participant element. In both cases the element uses a participationType “ENT” whose definition in HL7 V3 vocabulary is: “A person entering the data into the originating system. The data entry person is collected optionally for internal quality control purposes. This includes the transcriptionist for dictated text.”
APSR-10 – Observation related to multiple specimens: For example tumor staging requiring combining data from multiple specimens (e.g., a breast excision with positive margins followed by a re-excision with clear margins – in this case the tumor size may be a composite of measurements from both specimens. Another example – staging of ovarian carcinomas with multiple biopsies of pelvis, peritoneum, nodes, omentum, etc.). To accommodate these use cases, the specimen organizer is able to represent either a single specimen or a group of specimens investigated together. The specimen collection procedure nested in this organizer is repeatable to give the possibility to describe each of the specimens of the group.
APSR-11 – Derivative specimens. Specimens derived from primary specimens for ancillary studies, which may be sent to a reference lab or done in another part of the same institution, are included in the scope of this profile. The results produced on a derived specimen are attached to this specimen in the report. However the hierarchy of specimens is not explicitly represented in the report (see APSR-07), apart from being tracked in the “Procedure steps section” (see APSR-01).
APSR12 – Multipart report. In some cases the pathologist may create report(s) or report contents in a third-party application and embed, link, or refer to that separate report in the report produced by the LIS. This use case is natively taken care of by the underlying document sharing infrastructure: The profiles “Cross Enterprise Document Sharing” (XDS), “Cross Enterprise Document Media Interchange” (XDM) and “Cross Enterprise Document Reliable Interchange” (XDR) enable the sharing of a “submission set” which groups the collection of documents issued from a particular healthcare act. The APSR could be grouped with a DICOM Key Object Selection list (DICOM KOS) referring to the whole slide images representing the specimens investigated. It could also be grouped with a related report produced in some format by a third-party application. In addition to being in the same “submission set” these related documents or references to images can also be explicitly referred from within an entry of the CDA APSR, as a reference to an externalDocument, externalObservation, externalProcedure or externalAct element.
APSR-14 – Gaps in SNOMED CT: It is not straightforward to encode Anatomic Pathology observations and AP ancillary technique observations and their corresponding value sets described in Volume IV (Value Sets for APSR) using SNOMED CT concepts (missing concepts, issues of postcoordination versus precoordination). Moreover, SNOMED CT cannot be mandated by an IHE profile since the usage in production of this terminology is conditioned by a license, and many countries have not contracted this license yet. Thus these observations are identified with LOINC codes. Their value sets MAY be encoded using a temporary coding system built by the IHE Anatomic Pathology domain (PathLex - OID: 1.3.6.1.4.1.19376.1.8.2.1), or by any other locally agreed upon vocabulary.
APSR15 – Preadopting codes from upcoming releases of terminologies or value sets. For some AP observations, the value sets are changing regularly, which may bring the need for APSR producers to encode some observations using new codes approved by the source organization in a future version not available yet. This process is enabled by the other, specify mechanism described in volume 3.
APSR16 – Exportable human-readable summary of an AP report. Need for a "summary version" of the anatomic pathology report intended to be subsequently extracted for use in other medical documents. Thus when authors of other medical documents feel the need to include a segment such as "...the pathology report states '[...]'", the "summary version" of the report would ideally be included in the square brackets instead of the entire report including all of its sections. In the future it may be possible to generate and populate the summary version using the controlled vocabulary and discrete data elements used in the report. However, in order to allow the pathologist to control how the report is summarized, should we introduce an optional (free text) "summary version" section into the standard? This would encourage those who are interested to control concise versions of their reports, and on the flip side help the natural language processing algorithm developers. This need of a free text summary is addressed by an optional “Report Textual Summary” sub-section nested in the mandatory Diagnostic Conclusion section. This Report Textual Summary sub-section does not contain any entry.
VOLUME 1 - INTEGRATION PROFILES
1.7 History of Annual Changes
Append the following at the end of section 1.7
Scope of changes introduced in the current year:
- The Anatomic pathology Structured Reports (APSR) Content Profile extends the scope of the Pathology and Laboratory Medicine (PaLM) Technical Framework to the provision of persistent anatomic pathology and Cytopathology structured reports for various purposes such as care provision, care coordination, screening, and health surveillance.
1.12 Glossary
Add the following terms to the Glossary in section 1.12:
APSR Anatomic Pathology Structured Reports Content Profile
1.15 Scope of the Anatomic Pathology Technical Framework
Replace figure 1.15-1 by the one below
1.16 Anatomic Pathology Integration Profiles
Replace figure 1.16-1by the new one below, taking the new profile(s) into account.
1.17 Dependencies among Integration Profiles
Add the following lines to Table 1.17-1
| Anatomic Pathology Structured Reports to (APSR) | Cross-Enterprise DocumentSharing (XDS)in ITI-TF |
Implementers of APSR Content Profile may implement the XDS Profile to enable sharing of the pathology reports within an XDS Affinity Domain. In this case, the Content Creator actor must be grouped with an XDS Document Source actor, and the Content Consumer actor must be grouped with an XDS Document Consumer actor. |
Ensure that the sharing of laboratory reports within an XDS Affinity Domain can co-exist with the sharing of other types of documents |
| Anatomic Pathology Structured Reports to (APSR) | Cross-Enterprise DocumentMedia Interchange (XDM) in ITI-TF | Implementers of APSR Content Profile may implement the XDM Profile to enable sharing of the laboratory reports using media. In this case, the Content Creator must be grouped with an XDM Portable Media Creator and the Content Consumer with an XDM Portable Media Consumer. | Ensure that the
sharing of laboratory reports on media can co-exist with the sharing of other types of documents |
| Anatomic Pathology Structured Reports to (APSR) | Cross-Enterprise Document Reliable Interchange (XDR) in ITI-TF | Implementers of APSR Content Profile may implement the XDR Profile to enable sharing of the laboratory reports using reliable point-to-point network messages. In this case, the Content Creator must be grouped with an XDR Document Source, and the Content Consumer must be grouped with an XDR Document Recipient. | Ensure that the sharing of laboratory reports through reliable point-to-point messages can co-exist with the sharing of other types of documents |
1.18 Profiles Overview
Append sub-section 1.18.3 (takenfrom the current profile abstract) at the end of section 1.18.
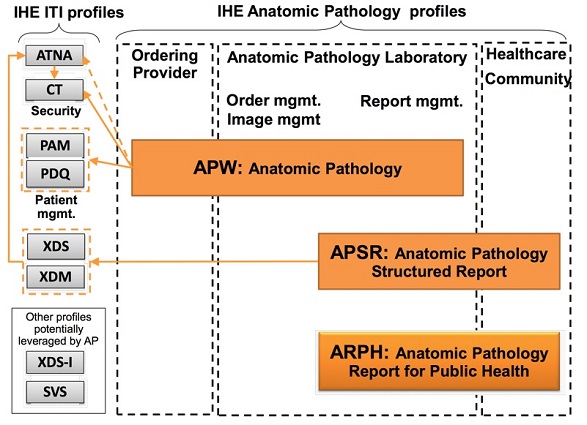
1.18.3 Anatomic Pathology Structured Reports (APSR)
This Content profile describes an anatomic pathology structured report (APSR) as a CDA r2 document to be published towards a document sharing resource such as a shared electronic health record used by a community of care providers, relying on one of the infrastructure document sharing/exchanging profiles defined in IHE ITI TF.
1.19 Actors Description
Add the following actors’ descriptions
Content Creator: An application responsible for the creation of content and transmission to a Content Consumer. This actor is involved in the APSR profile to issue anatomic pathology structured reports.
Content Consumer: An application responsible for viewing, importing, or other processing of content created by a Content Creator Actor. This actor is involved in the APSR profile to consume anatomic pathology structured reports.
Add Section 4 below, after the “ARPH integration profile” section.
4 Anatomic Pathology Structured Reports (APSR Profile
This Content profile describes an anatomic pathology structured report (APSR) as a CDA r2 document to be published towards a document sharing resource such as a shared electronic health record used by a community of care providers, relying on one of the infrastructure document sharing/exchanging profiles defined in IHE ITI TF.
Anatomicpathology reports (APR) document the pathologic findings in specimens removed from patients for diagnostic or therapeutic reasons. This information can be used for patient care, clinical research and epidemiology. Standardizing andcomputerizing APRs is necessary to improve the quality of reporting and the exchange of APR Information.
This Content profile describes an anatomic pathology structured report (APSR) as a CDA r2 document to be published towards a document sharing resource such as a shared electronic health record used by a community of care providers, relying on one of the infrastructure document sharing/exchanging profiles defined in IHE ITI TF.
Anatomic pathology reports (APR) document the pathologic findings in specimens removed from patients for diagnostic or therapeutic reasons. This information can be used for patient care, clinical research and epidemiology. Standardizing and computerizing APRs is necessary to improve the quality of reporting and the exchange of APR information.
The current scope of this IHE content profile covers APSR for surgical pathology in all fields of anatomic pathology (cancers, benign neoplasms as well as non-neoplastic conditions) as well as for Cytopathology. The profile handles information of “traditional” pathology observation using light microscopy (including immunohistochemistry, FISH, etc.).
Forensic pathology (autopsy, toxicology) will be addressed in further cycles as well as special ancillary techniques (e.g., flow cytometry, cytogenetics, electron microscopy).
Goldsmith provides recent recommendations that delineate the required, preferred, and optional elements which should be included in any APR for surgical pathology, regardless of report types.
Several international initiatives intend to define standard structured templates for specific types of APRs. In the cancer domain, in the United States, the CAP (College of American Pathologists) has published 80 cancer APSR templates (cancer checklists and background information)[1]. In France, the SFP (French society of pathology) [2] and the INCa (French National Cancer Institute) [3] have published 23 APSR templates of minimal set of data required for a primary tumor. In Australasia, the Royal College of Pathologists Australasia (RCPA) and the Cancer Australia developed an initial 6 reporting protocols (lung, melanoma, breast, colorectal, lymphoma and prostate) and a framework to guide development of the protocols, in partnership with national clinician and pathologist organizations. In some countries, the recommendations for generic and specific APSR requirements have become clinical guidelines, the use of which may be required by accrediting bodies.
This profile has also benefited from the guidance on cancer AP reports provided by the North-American Association of Central Cancer Registries; some of the example snippets captured in the profile leverage the NAACCR Standards for Cancer Registries, Volume V, Pathology Laboratory Electronic Reporting.
In addition to standardizing the cancer APR contents, it is necessary to computerize them. Several studies have focused on defining an appropriate IT standard comprising the structured and encoded clinical documents. Some of the implementation guides of APSRs proposed within these initiatives are not based on international healthcare IT standards (e.g., CAP eCC), other are based on either HL7 CDA r2 or CEN archetypes. HL7 CDA r2 is one of the most reliable standards that can support these needs. CDA allows the clinical data to be both machine and human-readable and provides a framework for incremental growth in the granularity of structured, codes-bound clinical information. This document takes into consideration current very few national CDA implementation guides for the APSR developed in Netherlands (National IT Institute for Healthcare in the Netherlands [www.nictiz.nl] and in Germany.
This content profile describes an anatomic pathology report shared in a human-readable format, which may include images. In addition, this electronic AP report SHALL contain anatomic pathology observations in a machine-readable format, to facilitate the integration of these observations in the database of a consumer system.
The definition of required, preferred and optional elements in this content profile is mainly based on Goldsmith, for generic surgical pathology APSR (regardless of report types) and, in the cancer domain, on standard structured templates provided by United States, the CAP (College of American Pathologists) [4], the SFP (French society of pathology) [5] and INCa (French National Cancer Institute) [6] and the Royal College of Pathologists Australasia (RCPA).
Structured reports are composed of a header, which provides the context of care (patient, care providers, pathologists, laboratories, order, act documented …), and a body. The latter provides the clinical information, which accompanied the order and specimens as well as the observations, findings and conclusions delivered by the pathologist after examination.
The anatomic pathology report described in this profile, with its set of anatomic pathology observations in a machine-readable format, MAY also be used to share historical results with appropriate content anonymization and patient identification pseudonymization to create shared distributed repositories of anatomic pathology information.
Both the header and the body provide human-readable information. The body is a hierarchy of sections. Each section presents its content in its text element, as human-readable text, possibly illustrated by some embedded images. This human-readable content, or a part of it, may also be present as machine-readable data coded with the appropriate terminologies (e.g., ICD-O-3, SNOMED CT, LOINC, ADICAP, etc.) in entry elements at the bottom of the section.
There are zero or more entry elements in a section. Each entry element carries the machine-readable data related to a single specimen or to a group of specimens observed together. The entry is then subdivided per problem investigated.
The text element of the section is supposed to reflect the same organization: per specimen, and then, per problem investigated on the specimen. The profile leaves the layout of the text element up to the Content Creator applications, or to further constraints brought by national extensions. However, given that the text element is usually composed of free text (e.g., dictated text), assembled with the text generated from the set of data, machine-encoded in the entry elements below, the Content Creator application MUST handle these two kinds of content, and provide a user interface, which precludes any confusion between them, both at creation time and update time (e.g., using forms with dedicated free text areas and distinct protected areas for coded fields).
4.1 APSR Actors/Transactions
This section references two other IHE Technical Frameworks:
- IT Infrastructure Technical Framework (ITI TF)
- Patient Care Coordination Technical Framework (PCC TF)
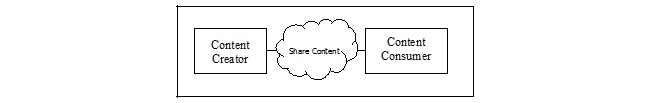
There are two actors in this profile, the Content Creator and the Content Consumer.
Content Creator: A Content Creator Actor is responsible for the creation of content and transmission to a Content Consumer.
Content Consumer: A Content Consumer Actor is responsible for viewing, import, or other processing of content created by a Content Creator Actor
Content (i.e., an anatomic pathology structured report) is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile. A Document Source or a Portable Media Creator may embody the Content Creator Actor. A Document Consumer, a Document Recipient or a Portable Media Importer may embody the Content Consumer Actor.
The sharing or transmission of anatomic pathology structured reports from one actor to the other is addressed by the use of appropriate content bindings with XDS, XDM or XDR integration profiles as explained in section 4 of Volume 3 of the Anatomic Pathology Technical Framework.
Figure 4.1-1 APSR Actor Diagram
4.1.1 Actor Descriptions and Requirements
This section is intentionally empty.
4.1.2 Document Content Modules
4.1.2.1 Anatomic Pathology Structured Report (APSR)
This document content module represents the generic set of constraints applied to any structured report for surgical pathology in all fields of anatomic pathology (cancers, benign neoplasms as well as non-neoplastic conditions) as well as for Cytopathology.
This document content module is identified by templateId 1.3.6.1.4.1.19376.1.8.1.1.1
The body of this document content module has the hierarchy of sections and entries depicted by figure 4.1.2.1-1. The only mandatory section is the diagnostic Conclusion Section. And the only mandatory entry is the Specimen Information Organizer Entry below this section.
Figure 4.1.2.1-1 Hierarchy of the body for APSR document content module
4.2 APSR Options
Table 4.2-1 summarizes the options that actors may take for this content profile. Dependencies between options when applicable are specified in notes. These options are summarized below the table, and further detailed in PCC TF-2, as indicated in the right column of the table.
| Actor | Options | Domain, Vol & Section |
|---|---|---|
| Content
Consumer |
View Option (1) | PCC TF-2:3.1.1 |
| Content
Consumer |
Document Import Option (1) | PCC TF-2:3.1.2 |
| Content
Consumer |
Section Import Option (1) | PCC TF-2:3.1.3 |
Note 1: The Actor shall support at least one of these options.
Grundlage / Basics
Grundlage dieses Konzeptes ist der Implementierungsleitfaden des Bundesverbands der Hersteller von IT-Lösungen für das Gesundheitswesen, e.V. (bvitg), Berlin, für den Arztbrief des deutschen Gesundheitswesens sowie der Diagnose- und Datentypleitfaden.
The APSR Germany is based on the trial implementation of the Bundesverband der Hersteller von IT-Lösungen für das Gesundheitswesen, e.V. (bvitg), Berlin, for the Discharge letter ("Arztbrief") of the German Health System, as well as the guidelines for diagnosis and data types.
- bvitG Arztbrief, v2.x, [CDAr2Arztbrief], 2012
- Diagnoseleitfaden v0.99b, 13.12.09
- Datentypleitfaden (HL7 V3)
Disclaimer
Dieses Dokument enthält keine komplette Spezifikation eines HL7 CDA R2 Arztbriefes bzw. Dokumentes. Es werden Teile eines Arztbriefes spezifiziert.
This document does not show a complete specification of a HL7 CDA R2 Discharge Letter. Parts of the Discharge letter are constrained.