HL7 CDA Core Principles
Inhaltsverzeichnis
- 1 Introduction
- 2 Common aspects
- 3 Data types
- 3.1 Introduction
- 3.2 XML representation of data types
- 3.3 Missing values (null flavor)
- 3.4 ANY as the generic data type
- 3.5 Boolean (BL)
- 3.6 Instance Identifier (II)
- 3.7 Encapsulated Data (ED)
- 3.8 Strings (ST)
- 3.9 Concept Descriptor (CD)
- 3.10 Coded with Equivalents (CE)
- 3.11 Coded Value (CV)
- 3.12 Coded Simple Value (CS)
- 3.13 Telecommunication Address (TEL)
- 3.14 Postal Address (AD)
- 3.15 Person Name (PN)
- 3.16 Organisation Name (ON)
- 3.17 Integer Number (INT)
- 3.18 Physical Quantities (PQ)
- 3.19 Point in Time (TS)
- 3.20 Intervals (IVL)
- 3.21 Interval of time (IVL_TS)
- 3.22 Interval of physical quantities (IVL_PQ)
- 3.23 Ratio of quantities (RTO_QTY_QTY)
- 4 Identification mechanisms
- 5 Vocabulary
- 6 Appendix
Introduction
This implementation guide specifies the Core Principles of using the Clinical Document Architecture [1].
Scope
This guide focuses on Data Types Release 1 [2]. Meanwhile an ISO specification ISO 21090 is out, also known as Data Type Release 2. The latter is subject to another im-plementation guide that will be published when ISO data types are used.
Focus of this document: HL7 Version 3 Data Types for CDA
The following chapters of this document will focus on the description of the data types and associated material. It will highlight the essentials in four chapters, all focusing on the Norwegian situation.
- Common aspects: explains common aspects of use of CDA and XML
- Data types: gives an overview and detailed specification of all data types used in CDA
- Identification schemes: explains major aspects of identification and the use in CDA
- Use of vocabulary: explains major aspects of vocabulary use in CDA.
Background
In many (European) countries, implementation guides for the use of data types in HL7 V3 are published. They take into account country specific constraints on the data types.
Legend of symbols
In this document several symbols are used.
Version history
| Version | Date | Description | Author |
|---|---|---|---|
| 1.1 | 2019-04-24 | Refurnished guide based on earlier work | Kai U. Heitmann |
Common aspects
Use of XML
HL7 Version 3 CDA uses the Extensible Markup Language (XML) as the exchange for-mat. It is assumed that the reader is familiar with common XML aspects. However, some constraints are made regarding the use of CDA.
Character Set
|
|
The character encoding of any CDA document shall be „UTF-8“. |
<?xml version="1.0" encoding="utf-8"?>
CDA document structure
The XML namespace for CDA Release 2 documents is urn:hl7-org:v3. This must be correctly mentioned in every XML instance. CDA XML documents starts with the root element ClinicalDocument.
<ClinicalDocument xmlns="urn:hl7-org:v3" xmlns:voc="urn:hl7-org:v3/voc"
xmlns:xsi="http://www.w3.org/2001/XMLSchemma-instance">
<!-- CDA Header -->
...
<!-- CDA Body -->
<component>
<structuredBody>
...
</structuredBody>
</component>
</ClinicalDocument>
Data types
Introduction
The following chapter describes the typical data types used in CDA implementation guides. For further information please refer to the HL7 V3 standard and its two parts concerning data types:
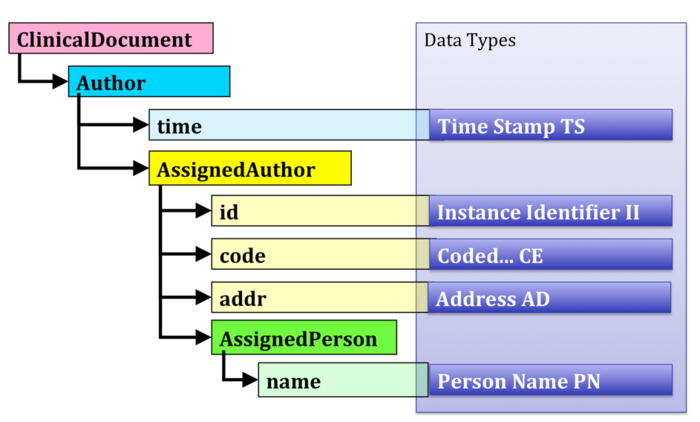
Data types are properties of model class attributes and the basic building blocks of in-formation in a CDA XML instance. For example, a model attribute named “time” has as its data type a time stamp “TS” (see Figure 1).
[Abbildung 1] CDA structure (left) and the data type specification (right)
XML representation of data types
Exceptions using XML element content
Combined data types
Conformance information
Missing values (null flavor)
ANY as the generic data type
Boolean (BL)
Instance Identifier (II)
Encapsulated Data (ED)
Strings (ST)
Concept Descriptor (CD)
Coded with Equivalents (CE)
Coded Value (CV)
Coded Simple Value (CS)
Telecommunication Address (TEL)
Postal Address (AD)
Person Name (PN)
Organisation Name (ON)
Integer Number (INT)
Physical Quantities (PQ)
Point in Time (TS)
Intervals (IVL)
Interval of time (IVL_TS)
Interval of physical quantities (IVL_PQ)
Ratio of quantities (RTO_QTY_QTY)
Identification mechanisms
Object Identifiers (OIDs)
Instance identification
Coding scheme identification
List of identification schemes
List of code systems
Vocabulary
Code Systems
Value Sets
Appendix
- ↑ Clinical Document Architecture Release 2 http://www.hl7.org
- ↑ HL7 Data Types Release 1 http://www.hl7.org
- ↑ HL7 Version 3 Abstract Data type definition http://www.hl7.org
- ↑ XML ITS for data types http://www.hl7.org
- ↑ CDA structure (left) and the data type specification (right)