Medikation-Section (Template)
(Teildokument von Übermittlung onkologischer Daten)
Foemig (Diskussion | Beiträge) (Die Seite wurde neu angelegt: „Medikation (Sektion) {{DocumentPart}} =Sektion: Medikation = {| class="hl7table" |bgcolor="ddddff"|Template ID|| colspan=2 | <OID für das Template> |- |bgco…“) |
Foemig (Diskussion | Beiträge) K |
||
| (4 dazwischenliegende Versionen desselben Benutzers werden nicht angezeigt) | |||
| Zeile 1: | Zeile 1: | ||
| − | |||
| − | |||
| − | |||
{{DocumentPart}} | {{DocumentPart}} | ||
| − | = | + | ==Section: Medikation == |
{| class="hl7table" | {| class="hl7table" | ||
| − | |bgcolor="ddddff"|Template | + | |bgcolor="ddddff"| || bgcolor="ddddff"| Template-Metadaten |
| + | |- | ||
| + | |bgcolor="ddddff"|Template-Typ|| Section | ||
| + | |- | ||
| + | |bgcolor="ddddff"|Template ID|| | ||
|- | |- | ||
| − | |bgcolor="ddddff"| | + | |bgcolor="ddddff"|generischeres Template || |
|- | |- | ||
| − | |bgcolor="ddddff"| | + | |bgcolor="ddddff"|genutztes Templates || |
|- | |- | ||
| − | | | + | |bgcolor="ddddff"|nutzende Templates || |
| + | |- | ||
| + | |bgcolor="ddddff"|abgeleitete Templates || | ||
| + | |- | ||
| + | |bgcolor="ddddff"|Generelle Beschreibung|| In diesem Abschnitt wird die Daten zur Medikation übermittelt. | ||
| + | |- | ||
| + | |bgcolor="ddddff"|allg. Erläuterung|| | ||
| + | |- | ||
| + | |bgcolor="ddddff"|Verhältnis zu IHE|| dt.Übersetzung oder Ergänzung oder neu | ||
| + | |- | ||
| + | |bgcolor="ddddff"|Ballotierungsstatus|| | ||
| + | |- | ||
| + | |bgcolor="ddddff"|Erweiterbarkeit|| offen oder geschlossen | ||
|} | |} | ||
| + | {{WorkBox| | ||
| + | Abgleich mit den anderen Medikation-Templates | ||
| + | }} | ||
| + | |||
| + | ===Modell=== | ||
[[file:Cdaonk_medikation2.gif|Medikation]] | [[file:Cdaonk_medikation2.gif|Medikation]] | ||
| Zeile 26: | Zeile 44: | ||
}} | }} | ||
| + | ===Attribute=== | ||
{| class="hl7table" | {| class="hl7table" | ||
! Lvl | ! Lvl | ||
! RIM | ! RIM | ||
| − | |||
! Name | ! Name | ||
| − | |||
! DT | ! DT | ||
! Kard | ! Kard | ||
| Zeile 37: | Zeile 54: | ||
! Beschreibung | ! Beschreibung | ||
| + | |- | ||
| + | |1 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |SubstanceAdministration | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | |2 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |@classCode | ||
| + | | | ||
| + | | | ||
| + | |fix | ||
| + | |SBADM | ||
| + | |||
| + | |- | ||
| + | |2 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |@moodCode | ||
| + | | | ||
| + | | | ||
| + | |fix | ||
| + | |ENV | ||
| + | |||
| + | |- | ||
| + | |2 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |templateID | ||
| + | | | ||
| + | | | ||
| + | |fix | ||
| + | |||
| + | |- | ||
| + | |2 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |id | ||
| + | |II | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | |2 | ||
| + | |bgcolor="ff8888"|act | ||
| + | |code | ||
| + | |CD | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | | 2 | ||
| + | |bgcolor="ccffff"| part | ||
| + | |consumable | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | | 2 | ||
| + | |bgcolor="ccffff"| part | ||
| + | |@typeCode | ||
| + | | | ||
| + | | | ||
| + | | fix | ||
| + | | CSM | ||
| + | |||
| + | |- | ||
| + | |3 | ||
| + | |bgcolor="ffff88"| role | ||
| + | |manufacturedProduct | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | |4 | ||
| + | |bgcolor="88ff88"|ent | ||
| + | |LabeledDrug | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
|- | |- | ||
|1 | |1 | ||
|bgcolor="ff8888"|act | |bgcolor="ff8888"|act | ||
| − | |||
| − | |||
| | | | ||
| | | | ||
| Zeile 52: | Zeile 155: | ||
| 2 | | 2 | ||
|bgcolor="ffaaaa"| rel | |bgcolor="ffaaaa"| rel | ||
| − | |||
| − | |||
| | | | ||
| | | | ||
| Zeile 63: | Zeile 164: | ||
| 1 | | 1 | ||
|bgcolor="ccffff"| part | |bgcolor="ccffff"| part | ||
| − | |||
| − | |||
| | | | ||
| | | | ||
| Zeile 74: | Zeile 173: | ||
| 2 | | 2 | ||
|bgcolor="ffff88"| role | |bgcolor="ffff88"| role | ||
| − | |||
| − | |||
| | | | ||
| | | | ||
| Zeile 85: | Zeile 182: | ||
|4 | |4 | ||
|bgcolor="88ff88"|ent | |bgcolor="88ff88"|ent | ||
| − | |||
| − | |||
| | | | ||
| | | | ||
| Zeile 95: | Zeile 190: | ||
|} | |} | ||
| + | ===Beispiel=== | ||
| + | Aus dem VHitG-Arztbrief: | ||
| − | |||
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
<substanceAdministration moodCode="EVN" classCode="SBADM" negationInd="true"> | <substanceAdministration moodCode="EVN" classCode="SBADM" negationInd="true"> | ||
| Zeile 168: | Zeile 264: | ||
| − | [[Kategorie: | + | [[Kategorie:CDA Section Level Template|Medikation (Section)]] |
| + | [[Kategorie:cdaonk|Medikation (Section)]] | ||
Aktuelle Version vom 2. Oktober 2013, 10:37 Uhr
Dieses Material ist Teil des Leitfadens Übermittlung onkologischer Daten.
|
Inhaltsverzeichnis
Section: Medikation
| Template-Metadaten | |
| Template-Typ | Section |
| Template ID | |
| generischeres Template | |
| genutztes Templates | |
| nutzende Templates | |
| abgeleitete Templates | |
| Generelle Beschreibung | In diesem Abschnitt wird die Daten zur Medikation übermittelt. |
| allg. Erläuterung | |
| Verhältnis zu IHE | dt.Übersetzung oder Ergänzung oder neu |
| Ballotierungsstatus | |
| Erweiterbarkeit | offen oder geschlossen |
|
|
Abgleich mit den anderen Medikation-Templates |
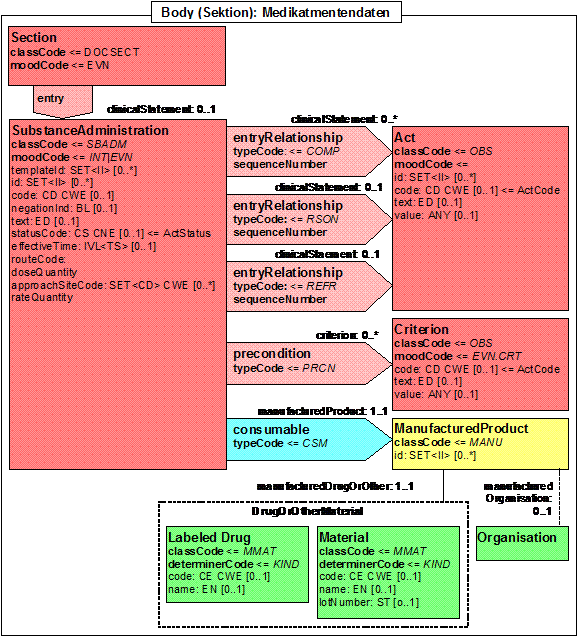
Modell
Abbildung 26: Medikation (gemäß IHE PCC)
|
|
Substance Administration Hinweis auf VHitG Addendum Medikation sowie epSOS!!! |
Attribute
| Lvl | RIM | Name | DT | Kard | Conf | Beschreibung |
|---|---|---|---|---|---|---|
| 1 | act | SubstanceAdministration | ||||
| 2 | act | @classCode | fix | SBADM | ||
| 2 | act | @moodCode | fix | ENV | ||
| 2 | act | templateID | fix | |||
| 2 | act | id | II | |||
| 2 | act | code | CD | |||
| 2 | part | consumable | ||||
| 2 | part | @typeCode | fix | CSM | ||
| 3 | role | manufacturedProduct | ||||
| 4 | ent | LabeledDrug | ||||
| 1 | act | |||||
| 2 | rel | |||||
| 1 | part | |||||
| 2 | role | |||||
| 4 | ent |
Beispiel
Aus dem VHitG-Arztbrief:
<substanceAdministration moodCode="EVN" classCode="SBADM" negationInd="true">
<templateId root="2.16.840.1.113883.10.20.5.6.42"/>
<consumable>
<manufacturedProduct classCode="MANU">
<manufacturedMaterial classCode="MMAT">
<code code="8611"
codeSystem="2.16.840.1.113883.6.88"
codeSystemName="RxNorm"
displayName="Povidone iodine"/>
</manufacturedMaterial>
</manufacturedProduct>
</consumable>
</substanceAdministration>
Aus IHE PCC TF 2:6.1.4.16.2
<substanceAdministration classCode="SBADM" moodCode="INT\|EVN">
<templateId root="2.16.840.1.113883.10.20.1.24"/>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.4.7"/>
<templateId root=""/>
<id root=\’\’ extension=\’\’/>
<code code=\’\’ codeSystem=\’\’ displayName=\’\’ codeSystemName=\’\’/>
<text><reference value=\’\#med-1\’/></text>
<statusCode code=\’completed\’/>
<effectiveTime xsi:type=\’IVL_TS\’>
<low value=\’\’/>
<high value=\’\’/>
</effectiveTime>
<effectiveTime operator=\’A\’ xsi:type=\’TS\|PIVL_TS\|EIVL_TS\|PIVL_PPD_TS\|SXPR_TS\’>
:
</effectiveTime>
<routeCode code=\’\’ codeSystem=\’\’ displayName=\’\’ codeSystemName=\’\’/>
<doseQuantity value=\’\’ unit=\’\’/>
<approachSiteCode code=\’\’ codeSystem=\’\’ displayName=\’\’ codeSystemName=\’\’/>
<rateQuantity value=\’\’ unit=\’\’/>
<consumable>
:
.
</consumable>
<!-- 0..\* entries describing the components -->
<entryRelationship typeCode=\’COMP\’ >
<sequenceNumber value=\’\’/>
</entryRelationship>
<!-- An optional entry relationship that indicates the the reason for use -->
<entryRelationship typeCode=\’RSON\’>
<act classCode=\’ACT\’ moodCode=\’EVN\’>
<templateId root=\’1.3.6.1.4.1.19376.1.5.3.1.4.4.1\’/>
<id root=\’\’ extension=\’\’/>
</act>
</entryRelationship>
<!-- An optional entry relationship that provides prescription activity -->
<entryRelationship typeCode=\’REFR\’>
<templateId root=\’1.3.6.1.4.1.19376.1.5.3.1.4.7.3\’/>
:
.
</entryRelationship>
<precondition>
<criterion>
<text><reference value=\’\’></text>
</criterion>
</precondition>
</substanceAdministation>